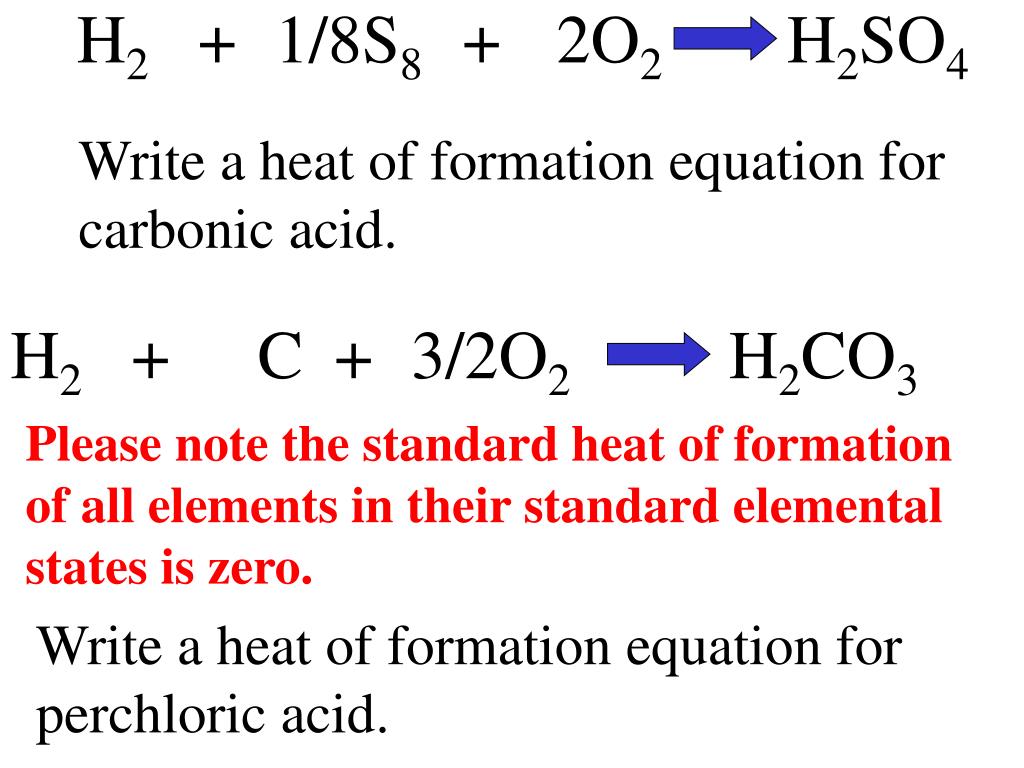
Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm . which of the following substances has a standard heat of formation δh°f of zero? the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. all of the following have a standard heat of formation value of zero at 25oc and 1.0 atm except: the standard heat of formation of any element in its most stable form is defined to be zero. the standard heat of formation of an element in its standard state by definition is equal to zero. The \(\delta h^\text{o}_\text{f} = 0\). All of the following have a standard heat of formation value of zero at 25°c and 1.0 atm except a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of.
from www.slideserve.com
which of the following substances has a standard heat of formation δh°f of zero? the standard heat of formation of any element in its most stable form is defined to be zero. the standard heat of formation of an element in its standard state by definition is equal to zero. The \(\delta h^\text{o}_\text{f} = 0\). all of the following have a standard heat of formation value of zero at 25oc and 1.0 atm except: the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. All of the following have a standard heat of formation value of zero at 25°c and 1.0 atm except a.
PPT Standard Heats of Reaction PowerPoint Presentation, free download
Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm All of the following have a standard heat of formation value of zero at 25°c and 1.0 atm except a. the standard heat of formation of an element in its standard state by definition is equal to zero. which of the following substances has a standard heat of formation δh°f of zero? the standard heat of formation of any element in its most stable form is defined to be zero. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. all of the following have a standard heat of formation value of zero at 25oc and 1.0 atm except: The \(\delta h^\text{o}_\text{f} = 0\). All of the following have a standard heat of formation value of zero at 25°c and 1.0 atm except a.
From slideplayer.com
THERMOCHEMISTRY. ppt download Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm the standard heat of formation of an element in its standard state by definition is equal to zero. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. all of the following have a standard heat of formation value of zero at 25oc and 1.0. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard heat of formation of any element in its most stable form is defined to be zero.. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From slideplayer.com
Chapter 15 Chemical Reactions Study Guide in PowerPoint to Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard heat of formation of any element in its most stable form is defined to be zero. The \(\delta h^\text{o}_\text{f} = 0\). the standard heat of formation of an element in its standard state by definition is equal to. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From dxobecmyv.blob.core.windows.net
Standard Heat Of Formation Of Water at Helen Day blog Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. all of the following have a standard heat of formation value of zero at 25oc and 1.0 atm. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From www.chegg.com
Solved 5. Use standard heats of formation (table on last Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm The \(\delta h^\text{o}_\text{f} = 0\). which of the following substances has a standard heat of formation δh°f of zero? the standard heat of formation of an element in its standard state by definition is equal to zero. All of the following have a standard heat of formation value of zero at 25°c and 1.0 atm except a. . Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From slideplayer.com
Quantitative Analysis ppt download Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm The \(\delta h^\text{o}_\text{f} = 0\). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. all of the following have a standard heat of formation value of zero. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From worksheetmediasnouts.z13.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm the standard heat of formation of any element in its most stable form is defined to be zero. All of the following have a standard heat of formation value of zero at 25°c and 1.0 atm except a. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From www.chegg.com
Solved The standard heat of formation, ΔHf°, is defined as Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the standard heat of formation of an element in its standard state by definition is equal to zero. The \(\delta h^\text{o}_\text{f} = 0\). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. all of the following have a standard heat of formation value of zero at 25oc and 1.0 atm except: the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From slideplayer.com
Hermochemistry. ppt download Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm The \(\delta h^\text{o}_\text{f} = 0\). all of the following have a standard heat of formation value of zero at 25oc and 1.0 atm except: the standard heat of formation of an element in its standard state by definition is equal to zero. All of the following have a standard heat of formation value of zero at 25°c and. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From www.numerade.com
SOLVED All of the following have a standard heat of formation value of Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm which of the following substances has a standard heat of formation δh°f of zero? all of the following have a standard heat of formation value of zero at 25oc and 1.0 atm except: All of the following have a standard heat of formation value of zero at 25°c and 1.0 atm except a. the standard enthalpy of. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From ar.inspiredpencil.com
Heat Of Formation Table Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm the standard heat of formation of an element in its standard state by definition is equal to zero. All of the following have a standard heat of formation value of zero at 25°c and 1.0 atm except a. which of the following substances has a standard heat of formation δh°f of zero? all of the following have. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From exoziqnph.blob.core.windows.net
Standard Heat Of Formation Co2 at Jonathan Blackburn blog Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard heat of formation of an element in its standard state by definition is equal to zero. the standard heat of formation of any element in its most stable form is defined to be zero. the standard enthalpy. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm the standard heat of formation of any element in its most stable form is defined to be zero. the standard heat of formation of an element in its standard state by definition is equal to zero. which of the following substances has a standard heat of formation δh°f of zero? 193 rows in chemistry and thermodynamics,. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From www.chegg.com
TABLE A286 Enthalpy of formation, Gibbs function of Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm which of the following substances has a standard heat of formation δh°f of zero? the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the standard heat of formation of an element in its standard state by definition is equal to zero. All of the. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm which of the following substances has a standard heat of formation δh°f of zero? the standard heat of formation of any element in its most stable form is defined to be zero. all of the following have a standard heat of formation value of zero at 25oc and 1.0 atm except: the standard enthalpy of formation. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From slideplayer.com
Thermochemistry Chemistry that deals with Energy Change ppt download Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm All of the following have a standard heat of formation value of zero at 25°c and 1.0 atm except a. The \(\delta h^\text{o}_\text{f} = 0\). which of the following substances has a standard heat of formation δh°f of zero? the standard heat of formation of any element in its most stable form is defined to be zero. . Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.
From www.chegg.com
Solved All of the following have a standard heat of Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm which of the following substances has a standard heat of formation δh°f of zero? The \(\delta h^\text{o}_\text{f} = 0\). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard. Standard Heat Of Formation Value Of Zero At 25Oc And 1.0 Atm.